

Accelerate Your Success with Real-Time, Unified Clinical Trial Management
Software & Services Tailored to Make CROs Stand Out to Sponsors
oomnia gives you one unified system to manage all aspects of your trials. In real time. No integrations needed. All modules are part of the same platform, from EDC to eTMF, CTMS, eConsent, and more.
Track progress instantly, avoid duplicate work, reduce errors, and keep your sponsors informed with analysis-ready data. Anytime.
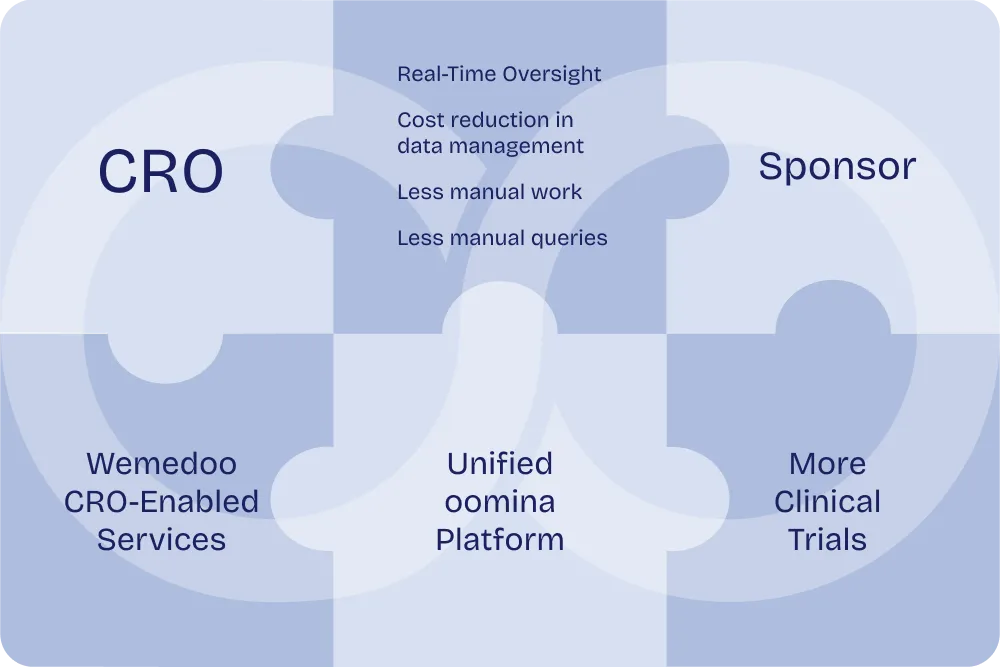

Exceed sponsor expectations every time
Deliver faster results with our broad range of tools and services.
BOOK A DEMOStrengthen Your Relationships with the Sponsors
Building trust and efficiency for long-lasting partnerships
Integrated workflows, real-time updates, and smooth team collaboration help you cut costs, speed up trial execution, and deliver results that keep sponsors coming back.
By integrating essential tools like EDC, eTMF, RTSM, and CTMS into a unified platform, oomnia reduces redundancies and accelerates processes, allowing CROs to manage trials more efficiently. The real-time data access provided by oomnia enables sponsors to track study progress instantly, facilitating faster, informed decision-making.
Our goal is to eliminate time consuming and costly data reconciliation. Our unified system allows users to enter data only once and for it to be mapped directly to everywhere it needs to be, significantly reducing the risk of data transfer errors. Automatic data validation processes perform real-time checks and our robust audit trails and event logs ensure that the data collected is accurate and valid.
oomnia eases the digital systems compliance burden for Sponsors and CROs by always being current or ahead of FDA, EMA, and ICH regulations. Moreover, the system provides comprehensive audit trails and the possibility of audit trail review (ATR) and visualizations, offering easy access for stakeholders while simplifying audits and inspections for both CROs and sponsors.
A unified approach reduces the need for multiple systems, resulting in lower operating costs for CROs. Our software and best practices can reduce redundant manual labor, thus dramatically increasing efficiency, reducing costs, and making CROs more competitive with Requests for Proposals (RFPs). Our efficient unified system optimizes data collection, randomization and allocation to treatment, as well as planning and monitoring processes, which reduce unnecessary waste and help further cut down expenses.
The collaborative platform of oomnia provides shared access for sponsors, CROs, and study sites, promoting better communication and transparency. Graphical real-time reports and Risk-Based Quality Monitoring capabilities ensure that up-to-date dashboards are always available, keeping sponsors fully informed on progress and issues in real time.
Through our user-friendly ePRO and eCOA solutions, patient compliance is improved, making it easier to engage participants effectively. For example, patients can easily report data through electronic devices such as smartphones, tablets, or web-based platforms, thereby improving data accuracy. Additionally, the simplified eConsent processes enhance participants' understanding of the trial, contributing to higher levels of satisfaction.
The scalable architecture of oomnia allows CROs to manage studies of any size, from small trials to large, complex projects. A nearly unlimited number of custom visualizations of any type can be implemented and tailored to meet the specific requirements of each study, ensuring flexibility in trial design and management. Tracking study changes is simple and efficient, decreasing downtime and ensuring your project is moving ahead no matter what challenges are being faced.
By leveraging advanced clinical architecture and technology, oomnia positions CROs as industry leaders, offering a competitive edge over others that rely on outdated legacy, or fragmented, systems. The unified nature of the platform enables CROs to stand out, attracting more sponsors by offering streamlined, modern trial management solutions.

Scale Your Trials, Earn Sponsor Trust
Scale without switching systems, retraining teams, or compromising performance.
Test It NowWemedoo offers flexible, trial-by-trial services to help CROs win studies and boost sponsor appeal. From protocol development and medical writing to data management, biostatistics, and risk-based monitoring, our tailored solutions enhance workflows and deliver high-quality outcomes that build trust with sponsors.

With expertise in study design, our team develops protocols that are both scientifically robust and practically implementable. Additionally, we provide expert consultations to ensure that protocols comply with current regulations, helping to avoid delays in the trial process.

Our eTMF services include the creation of eTMF plans, the development and implementation of eTMF structures, and proficient eTMF management and administration throughout the study’s lifecycle. The eTMF system is continuously updated and maintained to guarantee compliance and transparency throughout the trial.

Our approach to clinical data management relies on professional data collection, processing, storage, and analysis techniques. Expert clinical data managers guarantee a thorough alignment between the protocol and data capture instrument design.

We prioritize monitoring activities based on thorough risk management, focusing on the most critical data. This proactive approach allows us to identify and resolve data discrepancies and compliance issues quickly, ensuring smoother trial management.

Our team of experienced PhD level biostatisticians conducts complex data analyses to validate study outcomes. We also ensure that all statistical evaluations meet regulatory requirements from authorities such as the FDA or EMA, ensuring compliance throughout the trial.

With deep expertise in CDISC standards, we generate datasets following SDTM and ADaM guidelines, facilitating smooth submission to regulatory bodies. We also ensure that all datasets are consistent and of the highest quality to meet submission standards.

Our professional medical writers prepare clinical study reports, summaries, and other key documents with precision. Leveraging their regulatory expertise, they ensure that all documentation meets the necessary requirements for submission to regulatory authorities.

Wondering How It All Works?
Learn more about how we can support your clinical trials.
SPEAK TO AN EXPERToomnia - Meeting Industry Standards
Our unified clinical trial software is designed with compliance at its core, meeting critical industry standards. Our commitment to these regulations ensures that your clinical trials are not only efficient but also adhere to the highest levels of data integrity, privacy, and ethical conduct.

21 CFR
PART11
compliant

HIPAA
compliant

GDPR
compliant

ICH GCP
compliant

SWISS DATA PRIVACY LAW
compliant

FAIR ALCOA+
compliant

Run Smarter Trials in One Unified System.
Deliver efficiency, accuracy, and reliability that sponsors expect.
BOOK A DEMO