RTSM
Integrated in EDC. Automated. Reliable.
oomnia RTSM is part of one unified system. No switching, no delays, no gaps.
Simplify study logistics and drive faster, smarter trial outcomes
Integrated in EDC. Automated. Reliable.
oomnia RTSM is part of one unified system. No switching, no delays, no gaps.
Manage your study from one unified hub that combines EDC and RTSM. No switching between separate systems. With every function under one roof, you save time, reduce errors, and keep your trial on track.
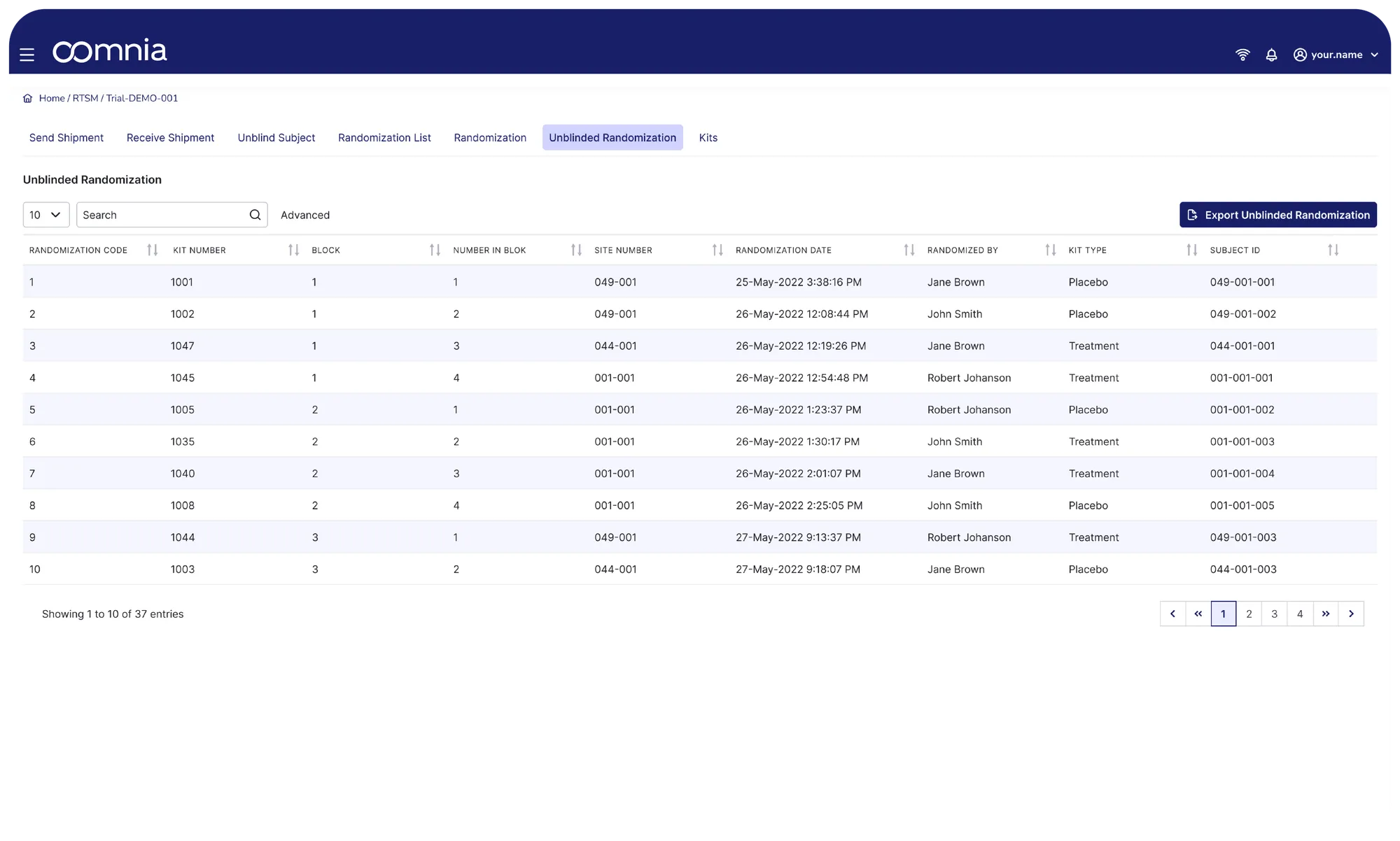
Randomize participants, verify IMP availability, assign the correct kit and allocate the study arm at a click of a button. All simultaneously and tracked in real time.


Randomize directly from the eCRF. With a single click.
We support various standard randomization approaches and algorithms. Our flexibility allows us to develop and validate any non-standard approach.
Want to randomize a device or an image reader? Our data model is flexible by design. It supports randomization of any object, not just participants.
Our unified database model allows direct communication between EDC and RTSM. Therefore, reconciliation is unnecessary, and manual work is eliminated.
With EDC and RTSM data within one database, you can control any aspect of clinical operations in real time. oomnia allows you to focus on critical elements and prevent protocol deviations.
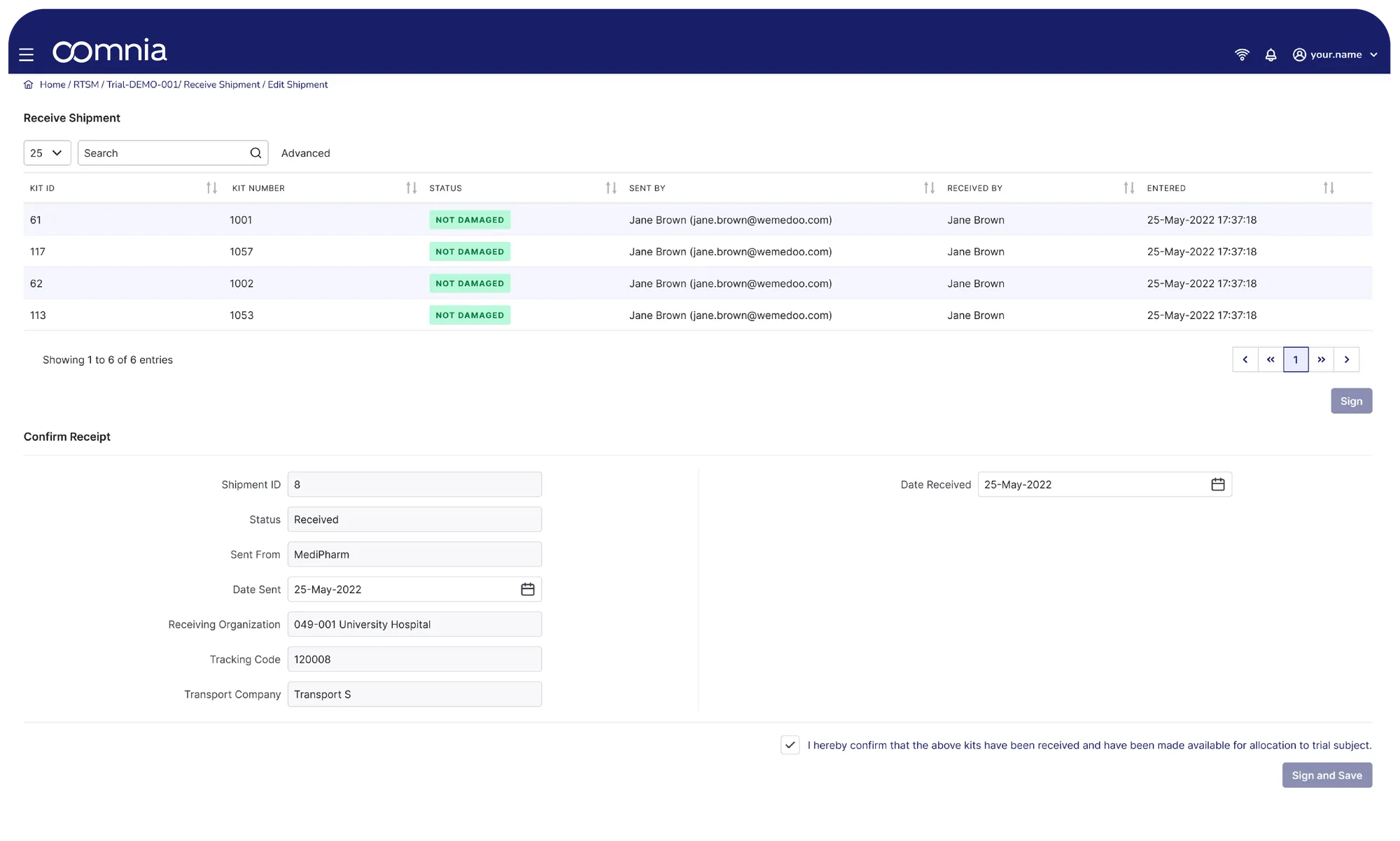
oomnia RTMS takes care of drug supply. oomnia power automation tools support you from organizational and oversight perspectives of drug supply.
oomnia RTSM for Smart Supply Logistics
Smart Alerts & Notifications
Receive instant notifications when inventory drops or nears expiry, keeping you in complete control.
Dynamic Expiry Protection
Automatically notify study stuff about soon-to-expire IMPs, ensuring patient safety and compliance. Block delivery of expired investigational products.
Instant, Accurate Kit Assignment
Eliminate manual checks with automated kit allocation that guarantees perfect treatment assignments every time.
Up To
73%
Cost Reduction Reports received by our clients based on reduction of manual labor and automated processed
> 50%
Operational Efficiency Based on client feedback linked to time efficiency gains
100%
Data Standardization




Instantly randomize participants and assign the right treatment kit, all in one click. Our system checks stock and expiry in real time to eliminate manual tasks and boost accuracy.
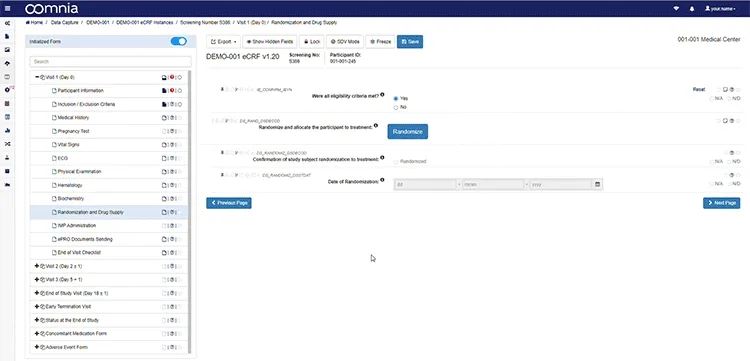
Easily configure any method (for example stratified, block, or dynamic) and upload lists with ease. Tailor your study’s randomization process to fit your unique trial design.
Handle complex scenarios with confidence, from object-level randomizations for images and samples to advanced study designs. Our platform covers every possibility for reliable results.

Inspired by the Latin Word omnia, Meaning 'All Things',
We Created oomnia.
A single, unified system that brings together every part of a clinical trial, from planning to global compliance.
EDC, RTSM, eTMF and CTMS, eSource, ePRO, eCOA and eConsent are integrative parts of one and the same ecosystem supporting multiple trials on a single instance.
Choose the modules and features that work for you and customize everything to fit your workflow.
oomnia RTSM Features Improve Your Clinical Research
Assign treatment groups with absolute precision
Control trial resources for smooth supply flow
Unify study data for actionable, instant insights
Guarantee full protocol adherence
Convert reports for fast analysis and seamless stakeholder collaboration

Looking to explore more? Get our RTSM flyer for more details.

Visit our blog for updates, insights, and event highlights.