


In the world of clinical studies, sponsors, CROs, and academic institutions often rely on multiple separated software tools that lack native integration. Wemedoo eliminates the chaos of disconnected trial software, making it a thing of the past.
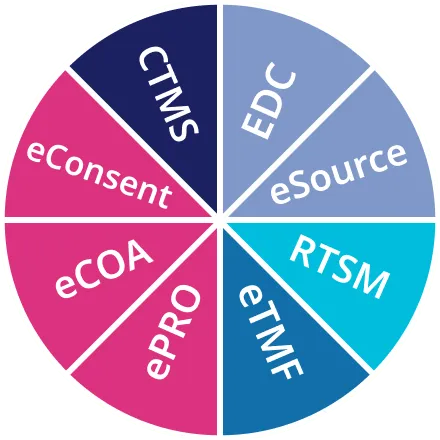
oomnia unifies all trial tools in one system: EDC with a drag and drop eCRF builder, eCOA, ePRO and eConsent for even more data entry points, a built-in RTSM, a fully fledged CTMS to overlook all trial data in real time, and an eTMF to meet all regulatory requirements. Unification of all trial tools means: one web-based login, one time data entry, one database, and one user interface for a smooth trial workflow.
oomnia combines all the essential features of individual tools, with additional capabilities. Add unlimited users, sites and trials, as well as real-time insights, and inbuilt AI features. Realize any trial type and scale your research to unprecedented levels.
Our Proof of Quality
We are ISO 9001 and ISO 27001 certified. Our commitment to robust information security & superior quality management is at the core of our innovative solutions, ensuring reliable & efficient trial management for our clients.



Trial efficiency goes up, while your costs go down. Reduce administration, programming, data reconciliation, data entry and analysis, and much more. Spend more time for quality assurance, reduce risks and improve your trial success rate.

Centralize & Simplify Clinical Trials
Unify your clinical trial tools on a single platform. Simplify data management and enhance collaboration now.
BOOK A DEMOExperience the benefits of a truly unified platform. Our major unification features bring together all the tools and data you need to streamline clinical trials and enhance collaboration. Explore the sections below for detailed information on how our unification capabilities can transform your research processes.
Our unified clinical trial software oomnia equips you with all the essential tools to manage clinical trials efficiently, streamline workflows, and boost productivity. It adapts to your specific needs, ensuring a smooth lifecycle from start to finish.
All necessary tools are accessible for managing clinical trials within a single software platform, reducing the need for multiple applications.
Looking for Clinical Trial Support That Fits Your Study?
Explore how our flexible, integrated services can help you accelerate timelines, improve quality, and reduce complexity.

Ensure consistency and integrity of your data. Having a single, unified database for clinical trials enhances data management, improves efficiency, and ensures data integrity.
oomnia allows to store all clinical trial data in a single, secure database to ensure consistency and ease of access.





Create a cohesive and efficient trial environment. A smooth workflow in clinical trial management is vital for maximizing efficiency and ensuring successful outcomes. See what key components oomnia offers to ensure a sleek workflow.
Our clinical trial software automates repetitive tasks such as patient scheduling, data entry, and monitoring alerts to enhance efficiency.
See oomnia in Action
Explore the full spectrum of our unified clinical trial tools through an insightful demo call conducted by our esteemed oomnia experts.