

All the Relevant Features at a Glance
Discover the Features of Our All-in-One Clinical Trial Software oomnia
Our clinical trial software oomnia redefines how research is conducted, offering unparalleled support through features like professional assistance and infinite trial types support. Foster universal collaboration with an accessible interface designed for researchers, clinicians, sponsors, and participants alike. Stay at the forefront of your trials with real-time reporting and scale your endeavors limitlessly with oomnia’s infinite trial scalability. Trust in oomnia’s unwavering commitment to the highest data security, ensuring the confidentiality of your trial data. Experience a future where each meticulously crafted feature amplifies your research impact. Ready to revolutionize your clinical trials? Explore the possibilities with oomnia today.


Make an Impact with oomnia
Work with oomnia and experience efficiency and precision like never before.
BOOK A DEMO
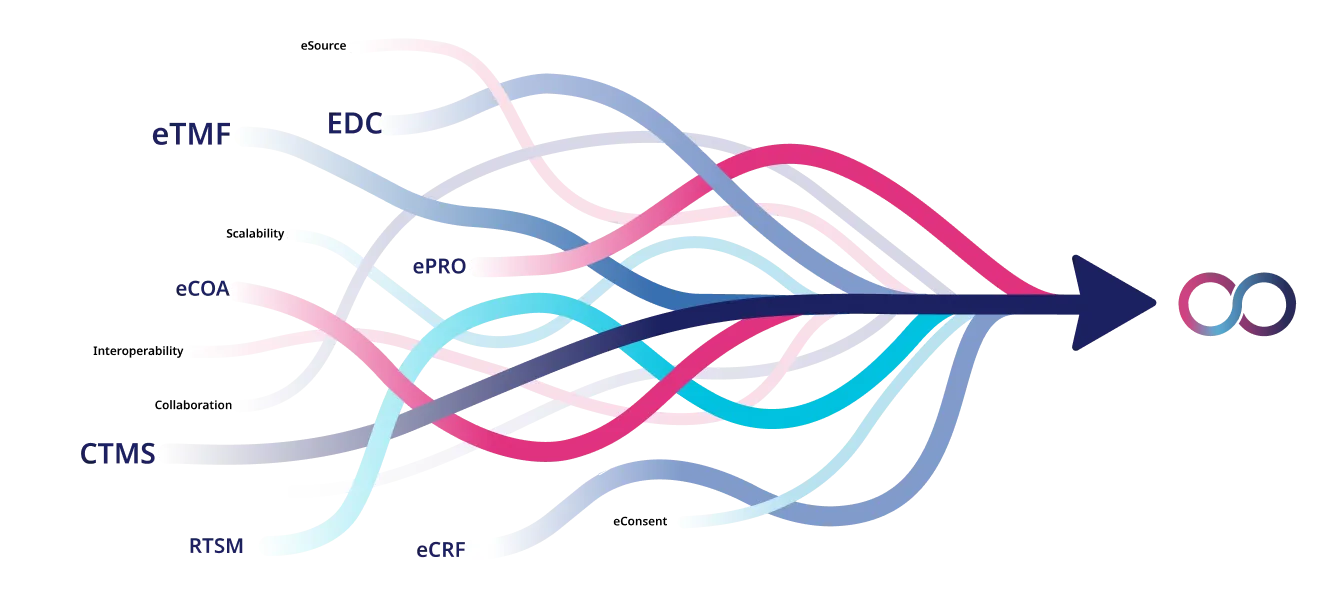
Why manage multiple tools when oomnia gives you everything in one go? oomnia changes the game by linking everything from EDC to eSource into one smooth, talking system. No more wasting time trying to get different software to play nice together. With oomnia, it is all built to work together from the start, making your trials faster, your data cleaner, and your budget happier. Dive into a world where every part of your clinical trial, from start to finish, just flows. Let oomnia show you how easy and efficient your research can be when everything communicates instantly and seamlessly. Ready to save time, boost data quality, and cut costs? Let us make it happen with oomnia.
LEARN MORE Learn about our Unified System
Why worry about the technical side of things when oomnia ensures you are always covered? oomnia is not just stable and user-friendly – it is backed by a team of professionals ready to help whenever you need it. Forget about the stress of troubleshooting; with up to 4 hours of professional support included in our subscriptions every month at no extra cost, you can focus on what truly matters to you. With oomnia, you are never alone; our experts stand ready to assist, ensuring your journey is smooth and uninterrupted. Embrace the confidence that comes with oomnia’s professional assistance, making your clinical research more efficient and less stressful. Let us keep your focus where it belongs – on groundbreaking research.
LEARN MORE Discover our Professional Assistance
With oomnia, you get to see what happens when all your data – from every tool, system, and institution – work together perfectly. No more data silos or headaches trying to match up information. oomnia is not just about gathering data; it is about making it easy to use, improving everything from how accurate and complete your data is to how well you meet regulations and get things done. Imagine a world where your clinical trials run smoother because all your information talks to each other, making teamwork better, speeding up your work, and helping you develop safe treatments faster. Get ready for a simpler way to handle your trials, where the power of connected data brings your research to life.
LEARN MORE Explore our Interoperability FeaturesOur Proof of Quality
We are ISO 9001 and ISO 27001 certified. Our commitment to robust information security & superior quality management is at the core of our innovative solutions, ensuring reliable & efficient trial management for our clients.



Ready for a solution that evolves with your trials? oomnia is here to ensure that your clinical trial software never poses obstacles to your clinical research, no matter the trial type. Whether it is decentralized, real-world data, or medical device trials – our solution seamlessly accommodates them all. We focus on integrating every aspect of your work smoothly for efficient management and maintaining flawless data integrity, compliance, and security. oomnia adapts to meet your unique needs, empowering you to focus on pioneering medical research. Step into a world where the type of trial you conduct amplifies your possibilities, not limits them.
LEARN MORE Explore Supported Clinical Trial Types
Need to keep your clinical trials moving, no matter where you are? oomnia keeps you connected to every crucial piece of your study – data, documents, insights, securely and efficiently, anytime and on any device. Even when you are offline or in the most remote locations without internet, our solution ensures you can continue your work seamlessly. Plus, with detailed audit trails and activity logs, you will always have a clear view of who did what and when, helping you stay on top of data integrity, compliance, and security. This is about enhancing teamwork, sharpening decisions, and ensuring smooth operations from planning to analysis. Welcome to a world where your physical location expands your research possibilities, not limits them.
LEARN MORE Learn About Remote Access Capabilities
Tired of the click marathon in your current clinical research tools? Dive into the simplicity of oomnia’s user-friendly interface, where we have cut down the unnecessary clutter and extra steps that bog down your workflow. Designed by and for clinical research experts, oomnia streamlines the process, allowing all stakeholders to navigate, manage, and make decisions with unprecedented ease. Stop endless clicks and welcome an intuitive experience that enhances efficiency and user satisfaction. With oomnia, we are not just reducing the click-burden – we are transforming how clinical trials are conducted, making it faster and more productive for everyone involved. Jump to a setup where everything is streamlined, making your research smoother and more productive.
LEARN MORE Discover Our Intuitive User Interface





Ever wished you could see your trial’s progress unfold in real time? With oomnia, that wish becomes reality. Our real-time reporting feature brings together data from every corner of your trial into a single, consistent, and compliant database. This means you get instant insights and analytics that were not possible before, allowing you to monitor progress, ensure patient safety, and evaluate outcomes as they happen. No more waiting for updates or guessing about your trial’s status. With oomnia, you are equipped to make quicker decisions and adapt your trial design on the fly, keeping your research agile and ahead of the curve. Join us in a new era of clinical trials, where real-time data puts you ahead of clinical research.
LEARN MORE Learn About Real-Time Reporting
Looking for a clinical trial solution that grows with your research? oomnia offers unparalleled scalability and adaptability, ready to meet the evolving demands of clinical trials, big or small. Our system seamlessly adjusts to fit your study’s scale, data diversity, and regulatory landscapes – from a compact exploratory study to a vast multinational trial. This built-in flexibility ensures that as your trial needs expand or shift, oomnia evolves right alongside, providing a stable foundation for growth. Scale your research infinitely with oomnia, where the size or complexity of your trial never limits your ambition.
LEARN MORE Discover Infinite Scalability Features
Need a solution where your data safety is never in question? In the digital world of clinical trials, data security must not be compromised. oomnia is armored with advanced security measures, encrypting and protecting every shred of data against unauthorized eyes. We recognize the trust you place in us with your sensitive information. That is why we have committed to the highest standards of data protection, ensuring your data is not just stored but shielded with the utmost care and state-of-the-art security. With oomnia, rest easy knowing your data is in safe hands, letting you focus on the groundbreaking research that drives progress.
LEARN MORE Explore Our Data Security Measuresoomnia - Meeting Industry Standards
Our unified clinical trial software is designed with compliance at its core, meeting critical industry standards. Our commitment to these regulations ensures that your clinical trials are not only efficient but also adhere to the highest levels of data integrity, privacy, and ethical conduct.

21 CFR
PART11
compliant

HIPAA
compliant

GDPR
compliant

ICH GCP
compliant

SWISS DATA PRIVACY LAW
compliant

FAIR ALCOA+
compliant

oomnia offers a centralized solution that not only streamlines communication among all stakeholders – investigators, study coordinators, sponsors, CROs – but also ensures instant access to data, given the right permissions. Our approach to collaboration eliminates manual, error-prone processes, speeding up decision-making, and enhancing transparency across the board. With instant communication and information consumption, knowledge sharing becomes effortless, enabling more informed decisions. Insights are readily available, helping to identify new opportunities in clinical research without the barriers of teams, organizations, or borders. oomnia is designed to break down these walls, making it easier to advance medicine by fostering a truly collaborative environment where progress is accelerated.
LEARN MORE Explore Seamless Collaboration Tools
Managing clinical trials can be complex, but our AI-powered features make it simpler and more efficient. Our platform integrates advanced tools to streamline data analysis, enhance decision-making, and reduce risks throughout the trial process. From predictive analytics that forecast potential outcomes to intelligent automation that simplifies patient selection and monitoring, our AI-driven features are designed to improve accuracy and efficiency. Whether it’s identifying trends or providing real-time insights, we ensure you stay one step ahead, allowing you to focus on delivering groundbreaking research with confidence. With AI on your side, your clinical trials become more precise, efficient, and impactful.
LEARN MORE Discover Our AI-Powered Features
Tailor every aspect of your trial management to fit your specific needs. Traditional systems force researchers to bend their workflows to fit pre-set structures. With us, oomnia molds to your requirements, allowing for on-the-fly adjustments. This means complex study settings can be mirrored and validated quickly, significantly cutting down the time it takes to get your study up and running. It offers template-based study design and configuration tools, enabling you to swiftly customize and reuse study templates, protocols, documents, and forms. This flexibility means you are not just working with what is available; you are shaping the system to meet your exact needs. With oomnia, experience a new level of customization that saves time and aligns perfectly with your research goals.
LEARN MORE Learn About oomnia's Customization Options