EDC
More than Traditional EDC. Built for the Trials of Any Complexity.
Experience high-performance data capture, smart automation, and seamless compliance in every trial.
Capture, manage and report clinical trial data
More than Traditional EDC. Built for the Trials of Any Complexity.
Experience high-performance data capture, smart automation, and seamless compliance in every trial.
See What is Happening in Your Trial as It Happens
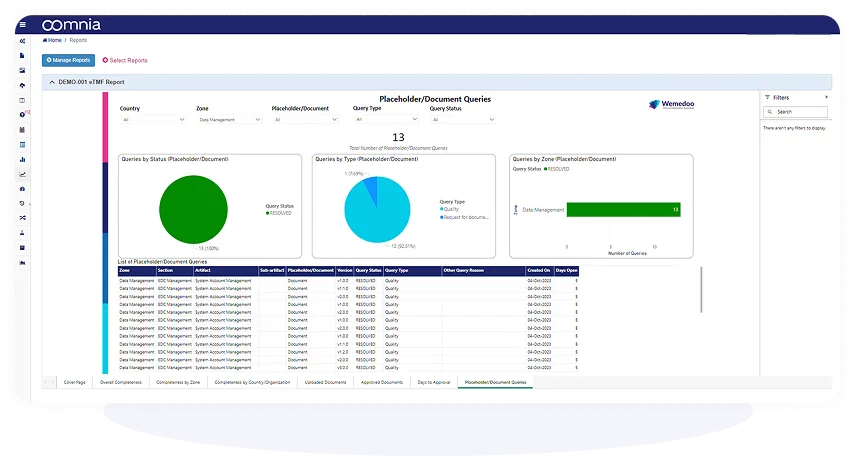
Generate custom reports, dashboards and statistical analyses directly within the system. All data is analysis-ready at any time and you decide what and how to present your data.
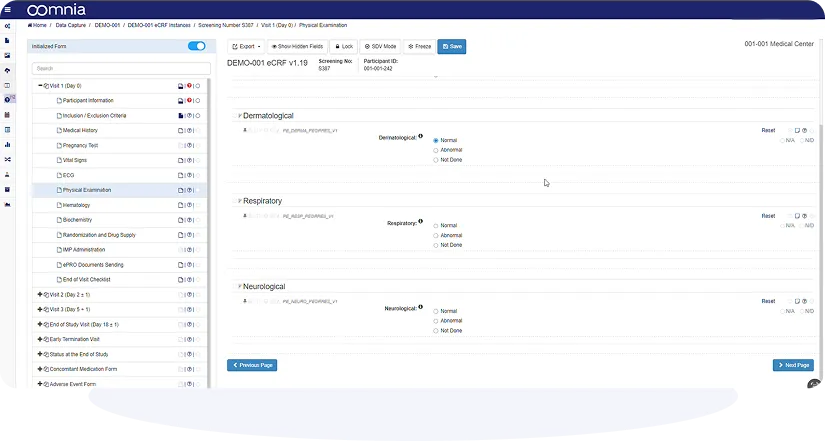
Positive user experience, intuitive design and powerful no-code user interface allows efficient workflow and productive collaboration.


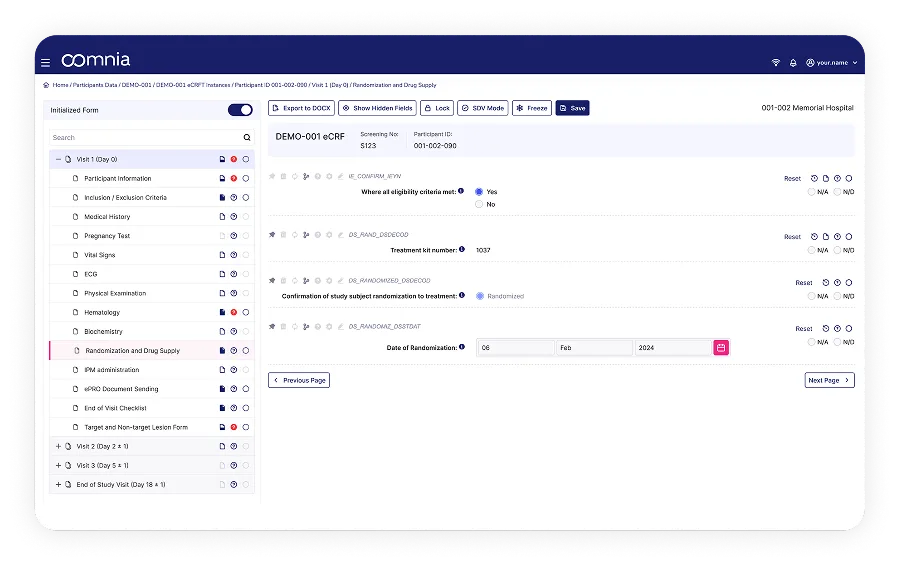
Get a clear, detailed view of every participants' journey. Track data entry, query resolution, SDV progress or study milestones in real-time.
Role-specific views show only what is needed. It streamlines tasks, reduces errors, and speeds up work.
Instant alerts for query responses, protocol deviations, and milestone deadlines gives you a complete oversight across trials.
Data flows directly into built-in analytics. It is always structured, always analysis-ready, no extra steps required.
Role-based access and intuitive navigation, boost confidence across teams.
Instant randomization of IP kits with automated tracking within the RTSM.

CRFs are created without programming. The intuitive, drag-and-drop interface, built-in templates, field validations, and logic rules simplify the setup process and ensure compliance from the start.
Tasks are automatically routed to the right users. Edit checks run in real time. Discrepancies are flagged instantly. Sites respond faster, monitors spend less time reviewing, and rework is reduced. Everyone sees only what they need to act.

Data is exportable in SDTM format and fully CDISC-compliant. No need for separate CDASH programming. This accelerates regulatory submission and saves months of manual preparation.
Up To
83%
Faster Trial Setup Client trial set up with 5000 questions compared to time needed by one of the biggest global CROs for the same trial in a different geography
100%
Real-Time Trial Oversight
100%
Client Retention





oomnia allows role-based permissions across all trials and oomnia modules. From data management to CRF setup and validation, all access levels are managed seamlessly with a single sign-on.
SAVE UP TO 75% OF NAVIGATION TIME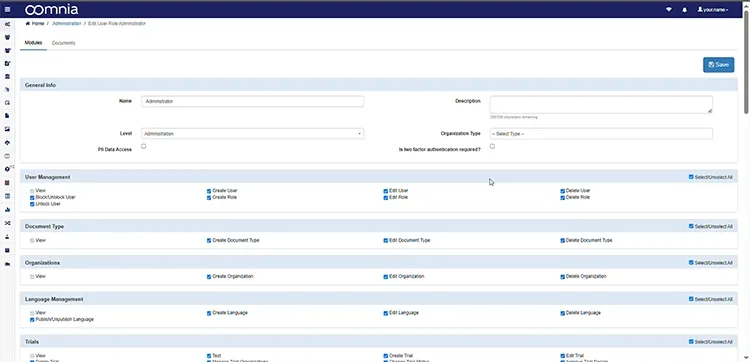
The system is based on intuitive drag-and-drop functionality, supporting the easy setup of highly complex configurations without programming.
0%PROGRAMMING REQUIRED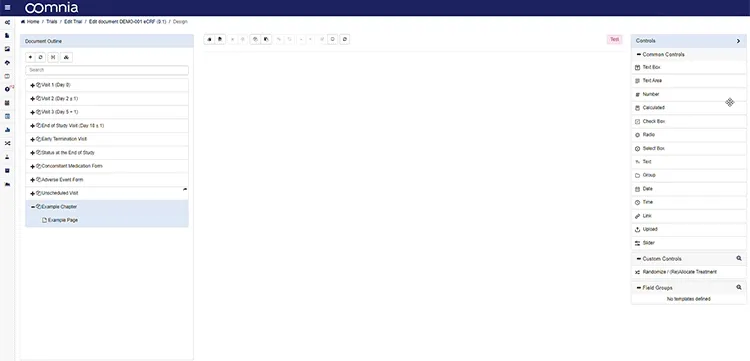
oomnia significantly reduces manual data cleaning, significant queries, and reconciliation work. Users report significant reductions in time spent resolving queries and managing SDV.
REDUCE UP TO 66%OF MANUAL QUERIES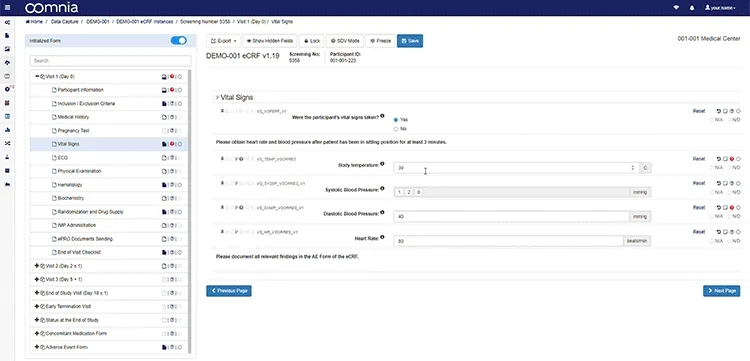
Inspired by the Latin Word omnia, Meaning 'All Things',
We Created oomnia.
A single, unified system that brings together every part of a clinical trial, from planning to global compliance.
EDC, RTSM, eTMF and CTMS, eSource, ePRO, eCOA and eConsent are integrative parts of one and the same ecosystem supporting multiple trials on a single instance.
Choose the modules and features that work for you and customize everything to fit your workflow.

Looking to explore more? Get our EDC flyer for more details.

Visit our blog for updates, insights, and event highlights.