ePRO
Real-Time ePROs for Smarter Clinical Trials
Collect patient feedback in real time & turn it into analysis-ready data.
Collect Patient Feedback in Real Time & Turn It Into Analysis-Ready Data.
Real-Time ePROs for Smarter Clinical Trials
Collect patient feedback in real time & turn it into analysis-ready data.

Deliver ePRO questionnaires in any format, length, or language. Easily tailor flows and dynamic responses to meet your study’s unique needs.
Access real-time, integrated graphical reports that combine eCRF and ePRO data for a comprehensive, on-the-spot trial overview.
Monitor data quality and patient compliance as it happens, enabling quicker, smarter decision-making with integrated real-time tracking.
Eliminate transcription errors with direct participant input and automated data aggregation for unmatched accuracy.
Enjoy a programming-free instrument setup with automatic ePRO delivery triggers based on eCRF completion. Accelerate your trial launch.
Reduce manual handling with automated data processing and built-in BYOD support, dramatically lowering your operational expenses.
Advanced Features of oomnia ePRO
oomnia ePRO makes data collection easy, accurate, and fully integrated. Patients complete questionnaires in real time from any device, while the system instantly connects their input to the study database. Trial directors, study coordinators, and biostatisticians, get better compliance, cleaner data, and faster decision-making.
Unlock the Full Potential of Your Clinical Trials

Inspired by the Latin Word omnia, Meaning 'All Things',
We Created oomnia.
A single, unified system that brings together every part of a clinical trial, from planning to global compliance.
EDC, RTSM, eTMF and CTMS, eSource, ePRO, eCOA and eConsent are integrative parts of one and the same ecosystem supporting multiple trials on a single instance.
Choose the modules and features that work for you and customize everything to fit your workflow.

Protect sensitive patient information with data encryption, multi-factor authentication, and strict role-based access controls.
SAVE UP TO 75% OF NAVIGATION TIME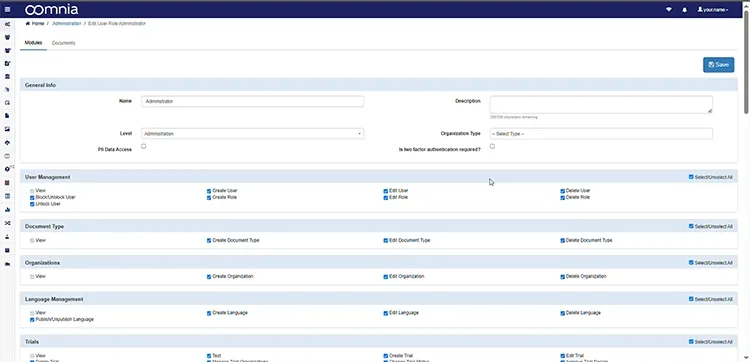
Tailor each interaction with an intuitive interface that adapts to individual needs, driving richer, more insightful responses.
0%PROGRAMMING REQUIRED
Send notifications to patients to complete their assessments or questionnaires, promoting timely data submission.
REDUCE UP TO 66%OF MANUAL QUERIESoomnia - Meeting Industry Standards
Our unified clinical trial software is designed with compliance at its core, meeting critical industry standards. Our commitment to these regulations ensures that your clinical trials are not only efficient but also adhere to the highest levels of data integrity, privacy, and ethical conduct.

21 CFR
PART11
compliant

HIPAA
compliant

GDPR
compliant

ICH GCP
compliant

SWISS DATA PRIVACY LAW
compliant

FAIR ALCOA+
compliant









Read more about the experiences from those who have partnered with us

All jobs, both large and small, are performed with the same dedication to quality, detail, innovative thinking, and rigor. It is always a pleasure to work with the Wemedoo team.
Georg Mathis, PhD
CEO Appletree CI Group AG

Wemedoo embodies all the traits desired in a partner in the clinical trial industry – expertise, innovation, reliability, compliance, and attention to detail.
Martina Diminic Smetisko, DMD
Managing Director at Marti Farm Ltd

Their dedication to, and support of, our projects has resulted in clear quality deliverables. They are always there to answer any questions with expertise in a timely manner. I am...
Their dedication to, and support of, our projects has resulted in clear quality deliverables. They are always there to answer any questions with expertise in a timely manner. I am looking forward to our next project together.
Volodymyr Stus, MD
Biopharmaceutics and Clinical Research Department Manager at Polpharma SA

Wemedoo's cross-functional, integrated teams capture EDC set-up, go-live, data capture, data cleaning, and finally data analysis in a globally compliant fashion supporting trials in different geographies including the US. Their...
Wemedoo's cross-functional, integrated teams capture EDC set-up, go-live, data capture, data cleaning, and finally data analysis in a globally compliant fashion supporting trials in different geographies including the US. Their speed of addressing customer needs sets in my personal experience industry benchmark whilst keeping these data solutions lean and affordable. Besides the great proprietary systems they use, their integrated team approach with personal responsibility and honest commitment to targeting outcomes differentiates the Wemedoo team from other providers. Wemedoo only made Oxular's key initial trial, a device-drug trial possible, as based on some pre-work, the Wemedoo team could substantially adapt and update the first EDC ideas for a trial under a US FDA IND. The Wemedoo team worked extremely focused on familiarizing US trial sites and clinical CROs with the use of their system, which turned out to be a key success factor of the respective trial. The highly professional and up-to-date capabilities in data sciences were mirrored by customer-centricity and super fast timelines of integrating mandatory adaptations to the data systems.
Friedrich Asmus, MD
Chief Medical Officer at Pykus Therapeutics

Wemedoo's technology, expertise, and intimate engagement with our team in the development of the EDC, site/patient interface, and metric capabilities are unique. They provided insight and challenged the underlying assumptions...
Wemedoo's technology, expertise, and intimate engagement with our team in the development of the EDC, site/patient interface, and metric capabilities are unique. They provided insight and challenged the underlying assumptions to ensure the study's goals and objectives were clearly defined and the operating structure matched with the study's intent. Their system is not a one-size-fits-all and they adapt to the needs of the sponsor, sites, physicians, and the study requirements into a system that merges health information/data and can then become not only informative but actionable.
James Kuras
Vice President of Clinical Affairs and Operations at CENTINEL SPINE
Our Proof of Quality
We are ISO 9001 and ISO 27001 certified. Our commitment to robust information security & superior quality management is at the core of our innovative solutions, ensuring reliable & efficient trial management for our clients.


THANK YOU FOR YOUR INTEREST
We have received your request and will get back to you as soon as possible for more details.

Get the answers you need about oomnia ePRO.
oomnia ePRO (electronic Patient Reported Outcomes) is a software solution where patients report outcomes related to their health condition and the impact of the treatment directly through electronic means. This can include various forms of technology like smartphones, tablets, computers, or specialized electronic devices. The oomnia ePRO system is designed to capture data in real time and can significantly enhance the quality and accuracy of patient‑reported data.
Our oomnia ePRO is easy to implement for sites and its intuitive user interface makes it extremely simple for participants of any background to use. Sponsors and study staff receive integrated real‑time insights based on participant questionnaires. Integration of oomnia ePRO with participant eCRFs or other data collection instruments also increases data quality and reduces time‑consuming, error‑prone, and costly reconciliations.
As oomnia ePRO works on any device with a simple mobile or desktop browser, participants don’t need trial‑specific devices or to install specialized software. There's no need for extensive training—if a device is lost or replaced, the participant can resume immediately via a secure link sent by SMS or email.
Patients can report data through electronic devices such as smartphones, tablets, or web‑based platforms, which is often more comfortable than paper diaries. Multilingual support allows patients to enter data in their preferred language, further improving engagement and adherence.
Initial delivery of ePRO questionnaires is triggered from within a participant's eCRF, and automated reminders prompt participants to complete their assessments. This ensures regular and timely data collection and encourages consistent participation.
Because oomnia ePRO is part of our unified system, data is automatically integrated into the trial database. Integrated graphical reports give study staff a real‑time overview of instrument completion, missing data, or lags in entry. The system can flag inconsistencies or outliers, allowing clinical teams to follow up with participants to clarify or correct any discrepancies.
To book a demo, simply click the button below, select a date in the booking tool, and fill in the required information. We will then send you an invitation to meet with an oomnia expert.

Looking to explore more? Get our ePRO flyer for more details.

Visit our blog for updates, insights, and event highlights.