

Interoperability
Running smooth clinical trials with our fully interoperable system. Easily connect oomnia with third party tools.


Running smooth clinical trials with our fully interoperable system. Easily connect oomnia with third party tools.

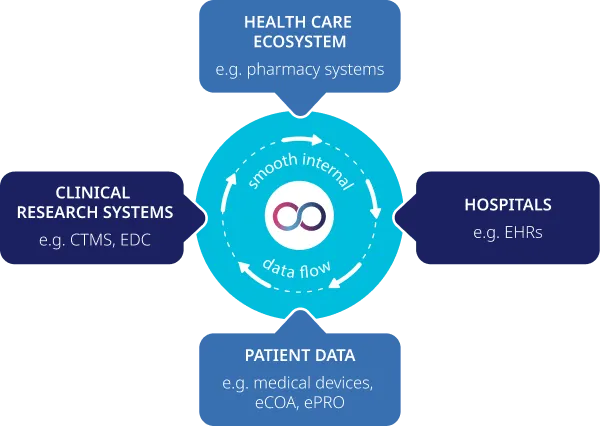
oomnia offers seamless data integration across systems. In today’s fast-paced world, seamless communication is crucial. The clinical trials sector often finds itself tangled in complexities that hinder smooth data flow and collaboration.
At Wemedoo, interoperability is not just a feature – it is in our DNA.
Our foundational innovation has transformed patient data acquisition, making it quicker, easier, and more accurate.

Unmatched Unification with oomnia
oomnia is the only platform that empowers you to connect instantly with all stakeholders across industries, trials, trial portfolios, and medical disciplines. An unmatched level of integration and efficiency is what sets us apart from the others.
BOOK A DEMOWe support all dimensions for your purposes. Our platform ensures complete interoperability across technical, semantic, organizational, legal, political, and social dimensions. Explore the sections below for detailed information on how we achieve seamless integration and collaboration in clinical trials.
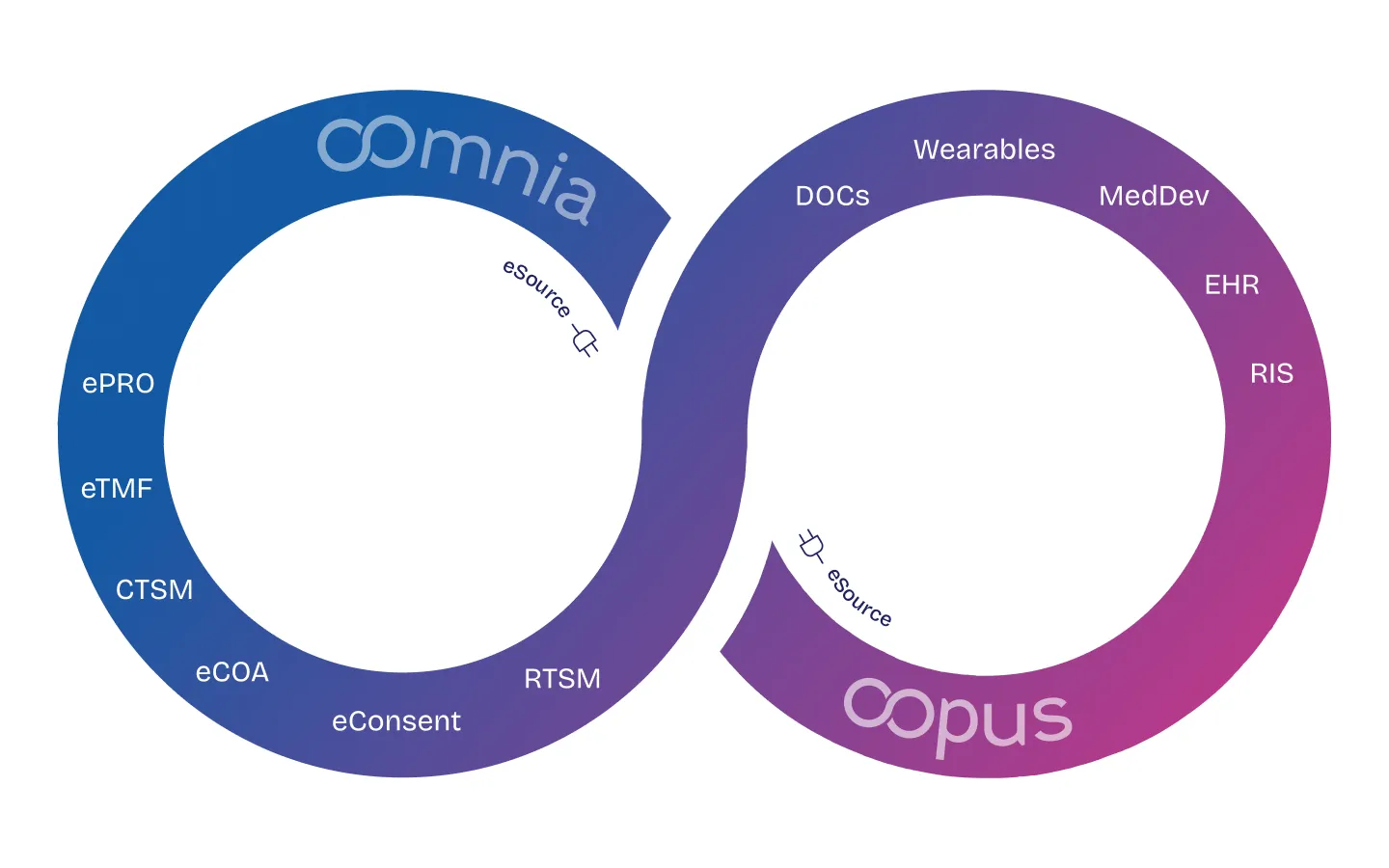
Enhance communication across your systems. oomnia ensures that different systems and software used in healthcare can communicate and work together effectively.
oomnia can exchange data through standard formats and protocols (e.g., HL7, FHIR, CDISC).
Our Proof of Quality
We are ISO 9001 and ISO 27001 certified. Our commitment to robust information security & superior quality management is at the core of our innovative solutions, ensuring reliable & efficient trial management for our clients.


Ensure consistent data interpretation and understanding. oomnia ensures that the data exchanged between different healthcare systems is shared accurately and understood consistently, which is vital for effective communication and collaboration in healthcare.
oomnia can exchange data through standard formats and protocols (e.g., HL7, FHIR, CDISC).





Bridge gaps in organizational workflows. Organizational interoperability is crucial for ensuring that different entities involved in clinical trials can work together seamlessly. oomnia focuses on aligning workflows and fostering effective collaboration among various stakeholders.
oomnia can exchange data through standard formats and protocols (e.g., HL7, FHIR, CDISC).
oomnia - Meeting Industry Standards
Our unified clinical trial software is designed with compliance at its core, meeting critical industry standards. Our commitment to these regulations ensures that your clinical trials are not only efficient but also adhere to the highest levels of data integrity, privacy, and ethical conduct.

21 CFR
PART11
compliant

HIPAA
compliant

GDPR
compliant

ICH GCP
compliant

SWISS DATA PRIVACY LAW
compliant

FAIR ALCOA+
compliant

Keep aligned with regulatory requirements. oomnia adheres to legal interoperability, which makes sure that all activities within the healthcare ecosystem comply with relevant laws and regulations, protecting patient data and maintaining the integrity of clinical trials.
oomnia can exchange data through standard formats and protocols (e.g., HL7, FHIR, CDISC).

Centralize & Simplify Clinical Trials
Unify your clinical trial tools on a single platform. Simplify data management and enhance collaboration now.
BOOK A DEMOAlign your operations with regulatory standards. Governmental interoperability, according to oomnia, involves aligning with regulatory frameworks and considering government policies to ensure smooth operation and collaboration across different jurisdictions in the healthcare ecosystem.
oomnia can exchange data through standard formats and protocols (e.g., HL7, FHIR, CDISC).
Reduce Costs & Time to Market
time for
