Article
From consent to compliance in patient-centric clinical trials
In clinical research, patient-centricity is often assumed but rarely examined in depth.
While a digital consent form or a well-designed application may give the impression of a patient-first approach, the real question goes deeper: Is your trial truly serving the patient consistently, from day one to the end?
Clinical trial participants expect more than access; they expect clarity, personalization, and a seamless digital experience. They are not passive subjects; they are informed contributors. So, if your study connects with the patient on day one but loses them by month six, can it really be called patient-centric?
This blog post explores what it means to put the patient at the center from initial consent to sustained compliance and how the right tools and strategies can turn vision into practice.
eConsent: The first step to trust
Digital consent is often the first point between the participant and the protocol. But real eConsent goes beyond a signature; this is a moment to inform, empower, and build trust.
Modern eConsent tools must be interactive, accessible, and clear. They should adapt content to different literacy levels, languages, and formats (videos, simple summaries). This changes consent into a process of understanding, not just agreement.
But once consent is given, how do we stay connected?
ePRO vs. eCOA: Same goal, different roles
Recording patients' experiences is crucial in order to help them during the experiment, but not all technologies are made equal.
- ePRO (electronic Patient-Reported Outcomes) captures direct input from the patient, like pain, fatigue, and mood.
- eCOA (electronic Clinical Outcome Assessments) is the more broader category. It includes ePRO, but also clinician assessments, caregiver observations, and performance-based measures.
Understanding the distinction matters. It influences how data is collected, how questions are formulated, and how results are interpreted.
A unified strategy for both enables more consistent insights and better alignment across teams.
Implementation in the real world: Technical and usability challenges
Designing a patient-first clinical trial is one thing; delivering it in practice is something else entirely.
Teams often face two major categories of challenges:
- Technical complexity: Integrating multiple digital systems such as ePRO tools, visiting calendars, and notification workflows.
- Usability concerns: Making sure that research teams and patients can engage with the technology without experiencing delays or training fatigue.
Coordinating both on-site and off-site assessments across multiple time zones, roles, and systems requires a unified solution, clear task arrangement, and flexibility to adapt as the study evolves.
Bridging these gaps takes time and close collaboration between product, clinical, and technical teams, especially in early-stage trials where tools are still being developed.
Oncology and eCOA: Where technology is critical
In oncology trials, patient experience is a core part of evaluating therapeutic success.
Clinical objectives like tumor size or survival rates are crucial, but they do not always provide the whole picture. Patients may exhibit some progress, but they may also be silently suffering side effects that affect their day-to-day activities. Traditional assessments frequently fail to notice this. That’s where eCOA becomes vital.
By combining multiple perspectives, patient-reported outcomes (ePRO), clinician assessments, caregiver input, and performance-based measures, eCOA provides a more human understanding of how a treatment truly affects quality of life.
In oncology studies, eCOA is especially important for:
- Tracking subtle shifts in pain, fatigue, or mobility over time
- Monitoring tolerance to long-term therapies
- Capturing data that informs not only regulators, but also treating physicians and patients themselves
When deployed thoughtfully, eCOA tools allow researchers to go beyond the clinic and listen to the patient’s experience in real time, in real life.
Even without specific names or examples, this section reinforces the idea that technology is not just collecting data; it’s amplifying the patient’s voice, especially in the most sensitive and complex therapeutic areas.
Patient burden: Technology as an ally, not an obstacle
Patients are often asked to complete multiple daily questionnaires, sometimes over months. Without thoughtful design, this can lead to a dropout.
Technology should make things easier, not harder.
- Mobile-first design ensures accessibility anytime, anywhere. Patients can complete study-related activities from their phone, without needing a computer or in person.
- Smart branching logic keeps things simple by only showing questions that truly apply to the patient.
- Friendly UI and reminders help patients stay on track.
- Simple feedback and small moments of encouragement help patients stay motivated.
And when patients feel heard, they are more likely to stay engaged.
From consent to commitment: It’s time to rethink “compliance”
When we talk about patient “compliance,” we often mean completing forms, attending visits, and following protocols. But real life isn’t a checklist.
Patients have lives, questions, and emotions. True commitment doesn’t come from pressure; it comes from feeling informed, supported, and respected. It’s not just about following instructions but understanding why they matter.
The real goal is to shift from administrative compliance to active engagement, with tools that adapt to human behavior, not fight it.
Here is what that engagement journey looks like:
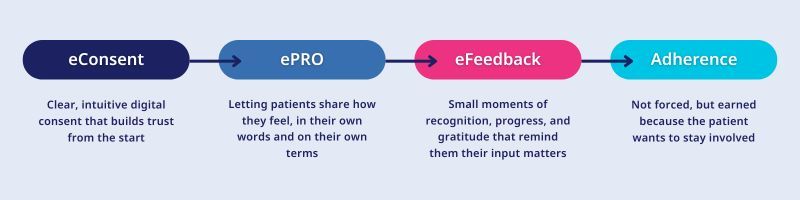
From vision to reality
A truly patient-centric trial isn’t defined by a single tool or a flashy feature. It’s defined by a continuous, connected journey where patients are informed, supported, and listened to every step of the way.
When technology fades into the background and human experience comes forward, you know you have done it right.
This is the vision Wemedoo builds toward, and oomnia is the solution designed to make it real. From interactive eConsent to real-time engagement tracking and seamless ePRO/eCOA integration, oomnia supports every step of the patient journey in one unified system.
Want to see how a truly unified, patient-centric journey works in practice?

October 9, 2024
Interoperability: The Missing Piece in Clinical Trial Efficiency
Blogs

February 4, 2025
Key Takeaways from EUCROF25: AI, Integration, and CRO Challenges in Clinical Trials
Blogs

January 31, 2025
The CRO’s Guide to Winning Sponsor Trust
Blogs