Software
Clinical knowledge at your fingertips.
Run the Study You Need.
Oomnia EDC is an integrated solution for collecting and reviewing clinical trial data. It is designed for all types of organizations regardless of their size to run any type of modern clinical trial they need.
We have developed Oomnia CRIS with sponsors, clinical research organizations (CROs), and academia in mind.
Benefits.
Cost Reduction.
Drastically lower clinical trial life cycle cost.
Time.
Shorten time to market.
Data.
Improve data quality and reuse it for other research work.
Solutions.
Cost Reduction.
Cloud-based EDC ecosystem creates synergies along the entire clinical trial life cycle.
Interoperability
Data interoperability allows seamless data exchange eliminates redundant and error-prone actions.
CDISC.
CDISC metadata standardization natively integrated.
Re-usability.
Reuse roles, settings, and variables easily across multiple trials./p>
Features.
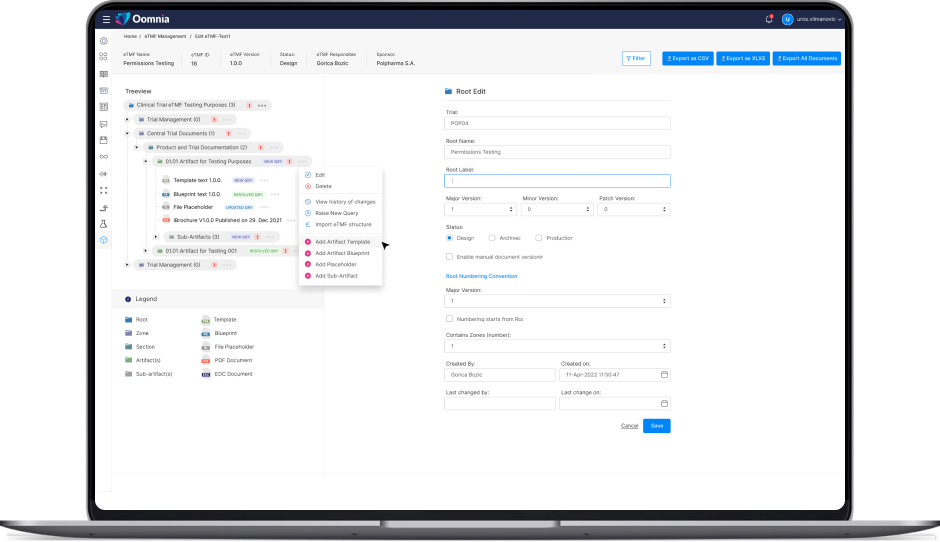
 Feature 1
Feature 1
Feature 1 description. Few sentences, maximum of 6 lines. Example see veeva.Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been the industry's standard dummy text ever since the 1500s
 Feature 3
Feature 3
Feature 1 description. Few sentences, maximum of 6 lines. Example see veeva.Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been the industry's standard dummy text ever since the 1500s
 Feature 5
Feature 5
Feature 1 description. Few sentences, maximum of 6 lines. Example see veeva (www.veeva.com/products/vault-edc)
 Feature 2
Feature 2
Feature 1 description. Few sentences, maximum of 6 lines. Example see veeva.Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been the industry's standard dummy text ever since the 1500s
 Feature 4
Feature 4
Feature 1 description. Few sentences, maximum of 6 lines. Example see veeva.Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been the industry's standard dummy text ever since the 1500s
 Feature 6
Feature 6
Feature 1 description. Few sentences, maximum of 6 lines. Example see veeva