Real-World Data
Your new world of knowledge

Discover patient insights and treatment outcomes in depth
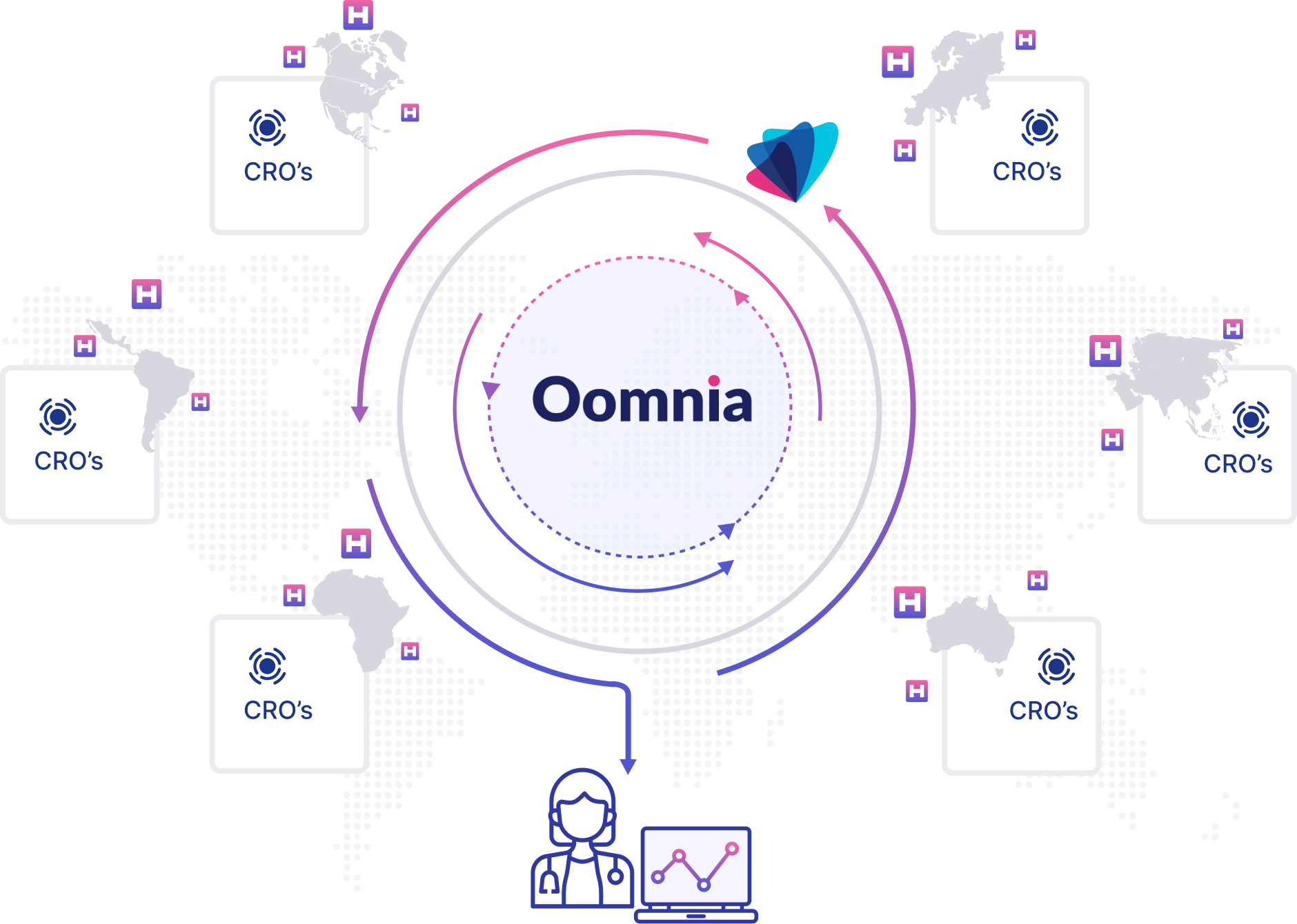
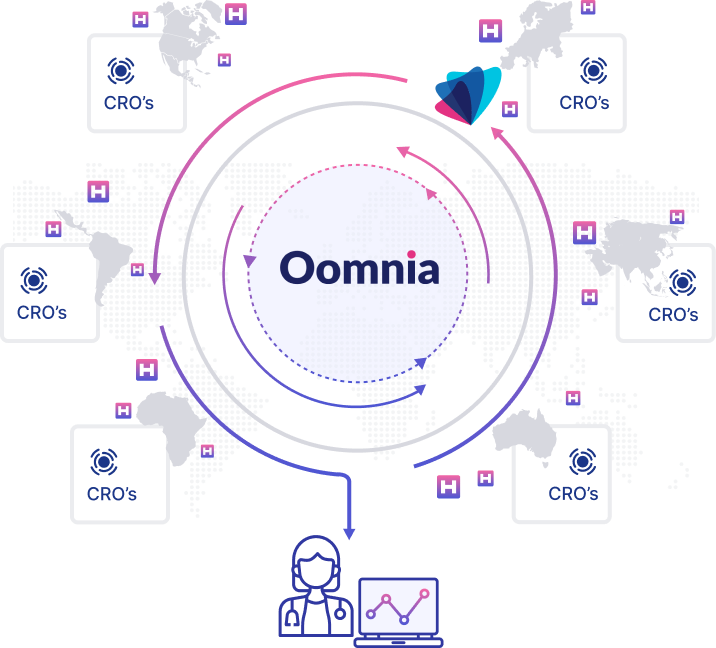
The Wemedoo Real-World Data Framework encompasses a complete ecosystem of expert services and software solutions dedicated to the collection, management, and analysis of real-world data.
Tap into patient experiences, outcomes, and shape evidence-based strategies that make a tangible difference in healthcare and medical research.
Benefits
Cost Reduction
Reduce research and product development cost
Acceleration
Accelerate development of treatments and interventions
Safety Increase
Improve safety assessments
Coverage
Expand coverage of rare diseases and conditions
Power your traditional, decentralized, or synthetic trials with unique real-world data collected by a global network of experienced clinical data professionals from a global, broad, and diverse patient population
27
CROs
99
Countries
>25
Therapeutic Areas
8,856
Sites
We provide custom validated real-world data from a uniquely broad and diverse population across countries on 5 continents to meet all client needs and requirements.
Treatment data are always connected to patient outcomes and direct access to patients is provided, both to ensure industry leading results.
With custom genetic analysis, general OMICS, and interpretation of medical imaging directly from all sites, unmatched real-world insights and evidence are ensured.
Base your studies on the best possible data and generate meaningful evidence and insights
Patient diversity and inclusion ensured, especially for rare diseases with small patient populations
Genomic profiling and advanced diagnostics available directly on most sites
Patient reported outcomes available for prospective studies
OMOP and CDISC compliance ensured
Semantic, syntactic and medical interoperability ensured
All data sets are fully machine readable
Patient consent is ensured for every data set if required
Regulatory compliance ensured in every country
Data collected with same standards as in clinical trials