Advanced randomization and supply management with oomnia RTSM
Randomize Participants Directly in the eCRF and Manage Supplies in Real-Time
- NAutomated randomization and IP allocation
- NEasy and real-time monitoring
- NEfficient blinding
- NControlled user access
Randomize Participants Directly in the eCRF and Manage Supplies in Real-Time
- NAutomated randomization and IP allocation
- NEasy and real-time monitoring
- NEfficient blinding
- NControlled user access
Discover the capabilities of oomnia RTSM firsthand
Unleash efficiency with oomnia rtsm
Revolutionize your trial management
Transforming the dynamics of clinical trial management, oomnia RTSM offers a comprehensive suite of functionalities tailored to the needs of modern clinical research. Our approach revolutionizes randomization, allocation to treatment, and trial supply management. As an integral part of our unified clinical research software, it automatically communicates with other modules enabling randomization and allocation to treatment directly within the eCRF. oomnia RTSM ensures precision, compliance, and superior quality in your clinical trials. Embrace the power of advanced technology and elevate your clinical research to new heights.
Benefits of our unified RTSM
Experience precision and efficiency
Experience the remarkable benefits of our unified oomnia RTSM tool, engineered to elevate your clinical trials with unparalleled precision and effectiveness. Explore the advantages of advanced randomization and supply management, combined with significant time and cost efficiencies, all while upholding the highest standards of quality. Discover how our RTSM solution is dedicated to optimizing timelines and trial management.
Advanced features of oomnia RTSM
Unlock the full potential of your clinical trials
Randomization
Transform your clinical trial dynamics
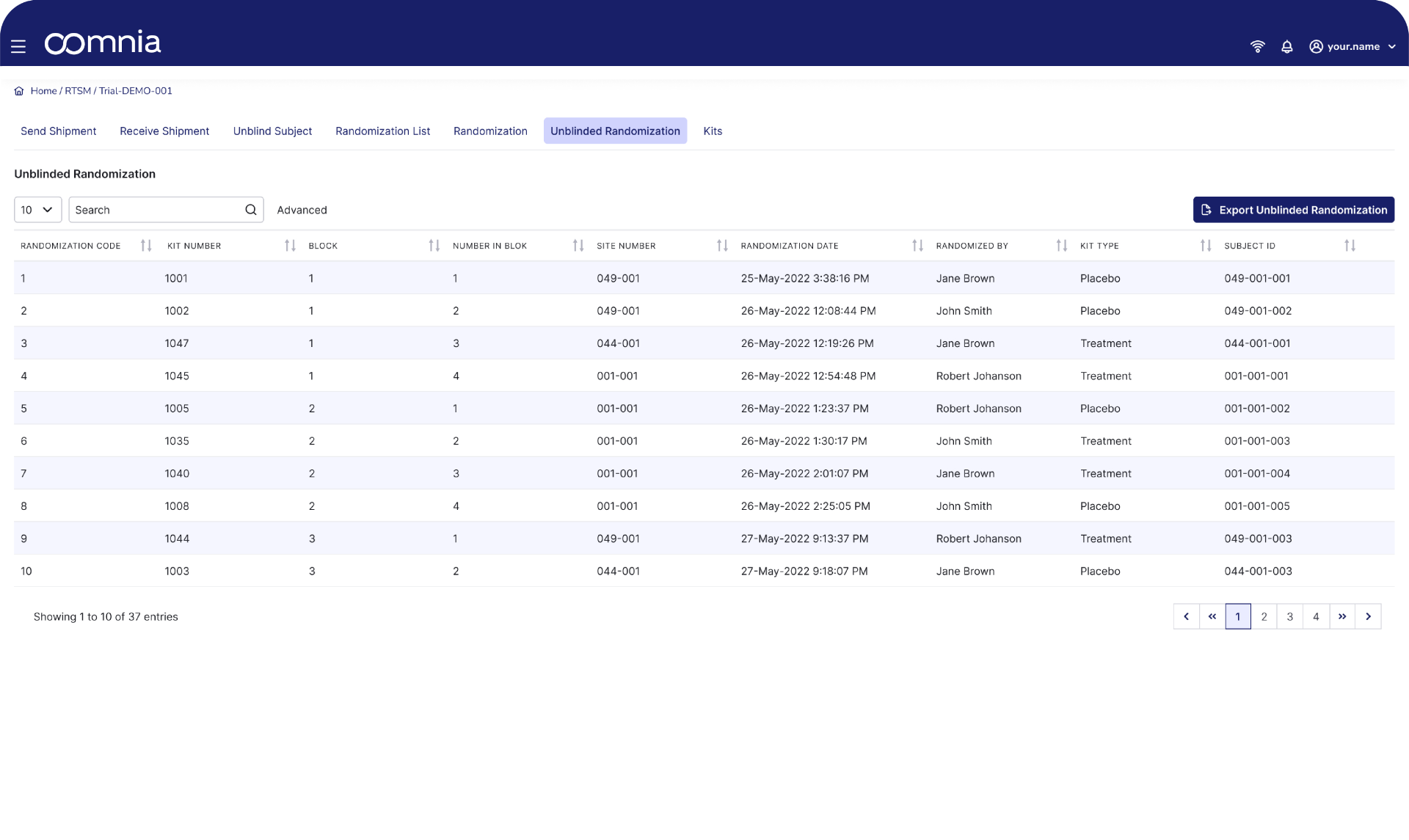
Randomization can be complex, especially for studies that consider factors such as stratification or adaptive study designs. This process is crucial to avoid bias and ensure the validity of study results. oomnia RTSM enables automated participant randomization and stratification, as well as allocation of treatment to study participants.
Dynamic randomization
- oomnia RTSM can support any randomization schedule
- Complex randomization schemes, like stratified or block randomization and dynamic assignment to treatment arms based on predefined criteria, are handled with ease
Patient stratification
- oomnia RTSM can automatically stratify participants based on predefined criteria such as country, site, age, sex, or any other characteristic entered into the eCRF
- Our reporting enables the study team to follow the stratification in real time
Automatic allocation to treatment
- Based on the randomization, oomnia RTSM can automatically present the appropriate IMP kit number to investigators directly in the eCRF
- The seamless nature of the system allows for real time tracking of IMP allocation, status, and easy reconciliation
Adaptive trial design
- For adaptive trials, randomization can be easily modified after interim data analysis and sample size re-assessment
- Adding randomization codes is as simple as uploading them to the system
Dynamic randomization
- oomnia RTSM can support any randomization schedule
- Complex randomization schemes, like stratified or block randomization and dynamic assignment to treatment arms based on predefined criteria, are handled with ease
Patient stratification
- oomnia RTSM can automatically stratify participants based on predefined criteria such as country, site, age, sex, or any other characteristic entered into the eCRF
- Our reporting enables the study team to follow the stratification in real time
Automatic allocation to treatment
- Based on the randomization, oomnia RTSM can automatically present the appropriate IMP kit number to investigators directly in the eCRF
- The seamless nature of the system allows for real time tracking of IMP allocation, status, and easy reconciliation
Adaptive trial design
- For adaptive trials, randomization can be easily modified after interim data analysis and sample size re-assessment
- Adding randomization codes is as simple as uploading them to the system
Management of study medication and materials
Simplify medication logistics
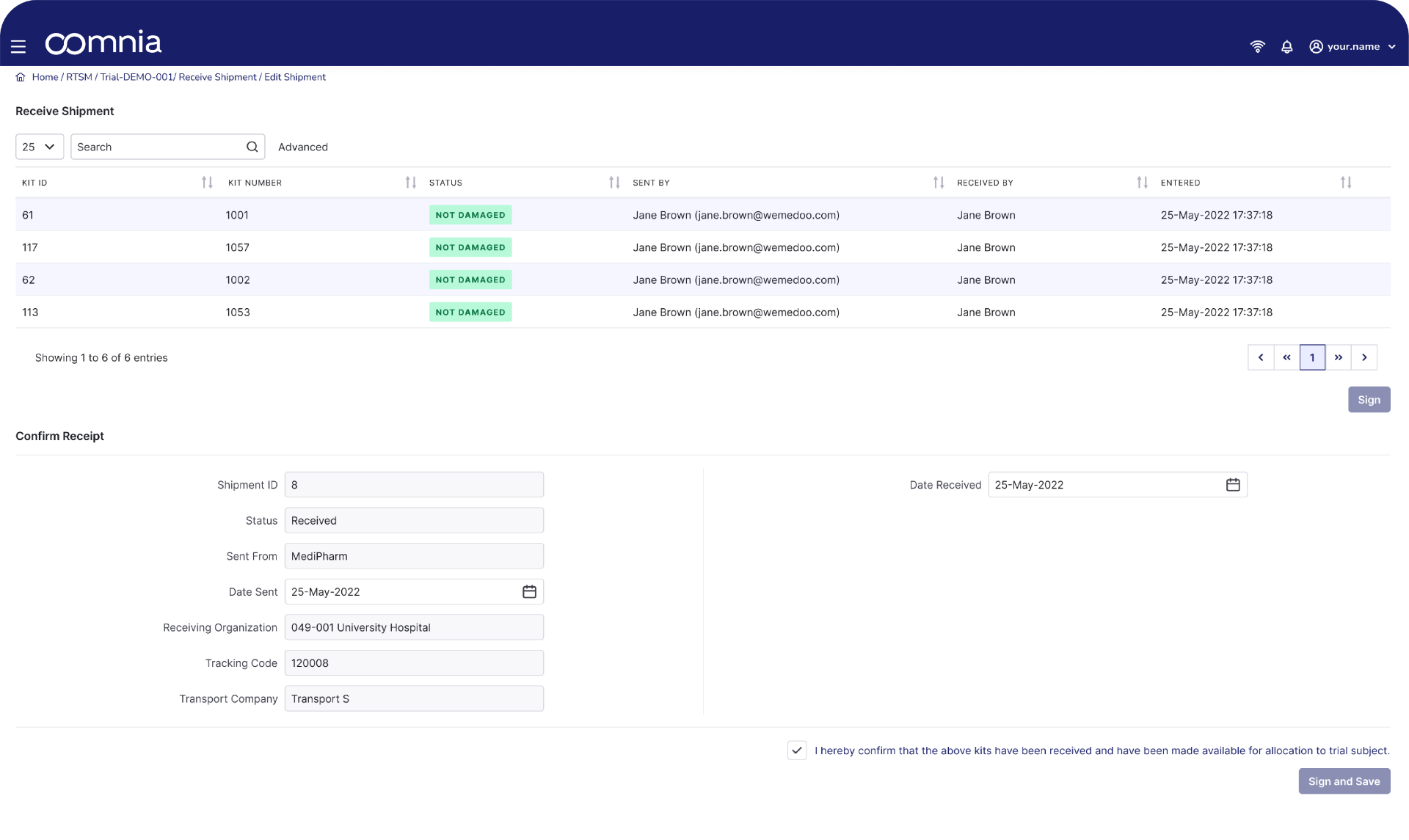
Our oomnia RTSM tool enables tracking of the distribution of IMP and study materials. This ensures that the right IMP kits have been delivered to study sites in the correct quantities and at the right time.
Integrated inventory management
- oomnia RTSM provides real-time tracking and management of IMP inventory at each investigational site and depot, ensuring adequate supply throughout the trial
IMP dispensation and return tracking
- The system tracks the dispensation of study drugs to participants and monitors the return of unused medication, maintaining detailed accountability logs
- Real-time reporting of IMP kit status and location
Automated reorder triggers
- Based on predefined thresholds, the system automatically notifies when stock levels are low to prevent shortages
- oomnia RTSM allows you to send IMP from one site to another with ease
Expiration date tracking
- oomnia RTSM keeps track of expiration dates of medications and materials
- The system prevents allocation of IMP kits close to their expiration date, ensuring no expired IMP is dispensed to participants
Integrated inventory management
- oomnia RTSM provides real-time tracking and management of IMP inventory at each investigational site and depot, ensuring adequate supply throughout the trial
IMP Dispensation and return tracking
- The system tracks the dispensation of study drugs to participants and monitors the return of unused medication, maintaining detailed accountability logs
- Real-time reporting of IMP kit status and location
Automated reorder triggers
- Based on predefined thresholds, the system automatically notifies when stock levels are low to prevent shortages
- oomnia RTSM allows you to send IMP from one site to another with ease
Expiration date tracking
- oomnia RTSM keeps track of expiration dates of medications and materials
- The system prevents allocation of IMP kits close to their expiration date, ensuring no expired IMP is dispensed to participants
Why oomnia
Integrated EDC, RTSM, ePRO, eCOA, eSource, CTMS, and eTMF
- Unified SaaS-solution
- Real-time analytics
- Full interoperability
- Unmatched user experience
- Easy learning curve
- Adaptive flexibility
- Exceptional services
- Instant collaboration
- Swift validation process
Blinding
Elevate the integrity of your clinical trials
Secure and integrated blinding mechanism
- oomnia RTSM ensures that all stakeholders are blinded to participant randomization to study arms
- Only IMP kit numbers are presented to study staff within the eCRF
- Automatic tracking and reporting on randomization and allocation to strata
Controlled access to unblinded data
- oomnia RTSM ensures only authorized staff can access unblinded information
- The system provides a robust audit trail and promotes transparency and accountability in the unblinding process
- Immediate unblinding notifications to study management
Data integration and reporting
Unlock seamless data flow
Our oomnia RTSM tool can retrieve data with other systems such as EDC (Electronic Data Capture) to ensure seamless data flow and consistent data recording. They also enable the generation of reports to monitor randomization and material management.
Centralized data access
Customizable reporting tools
- Customizable reports to meet specific trial requirements
- This includes the ability to select specific data points and metrics for tailored analysis and reporting
Real-time data reporting
- oomnia RTSM generates any report around any given parameter
- Generate reports on a patient-by-patient basis as required for filing in the eTMF
Data export capabilities
- Export data in various formats to facilitate further analysis or sharing with external stakeholders or regulatory bodies
Centralized data access
Customizable reporting tools
- Customizable reports to meet specific trial requirements
- This includes the ability to select specific data points and metrics for tailored analysis and reporting
Real-time data reporting
- oomnia RTSM generates any report around any given parameter
- Generate reports on a patient-by-patient basis as required for filing in the eTMF
Data export capabilities
- Export data in various formats to facilitate further analysis or sharing with external stakeholders or regulatory bodies
Compliance and blinding security
Safeguard your trial’s credibility
Regulatory compliance
- Our oomnia software is compliant with rigorous standards set by regulatory authorities like the FDA 21 CRF Part 11 and EMA EU GMP Annex 11
- oomnia undergoes constant validation, as well as third party assessments
Maintenance of the blind
- Blinding information is always kept secure and separate, in a manner that is impossible for even Wemedoo staff to discern
- Rigorous testing and validation has been performed for all RTSM functions to ensure sensitive blinding data cannot be leaked
trusted by








Complementary services
Unlock more possibilities
Our professional trial services complement your expertise. We support you with optimal trial implementation, management, and finalization. For RTSM, we are offering our biostatistics and statistical programming service. We are ready to work hand in hand with your team, as needed.
Biostatistics and statistical programming
Before the study’s commencement, biostatistical methods are used to oversee the study design in terms of calculating the sample size, and providing randomization solutions to compose a scientifically sound performance. As the data collection is complete and clinical study brought to a close, biostatistics provide a quantitative framework for interpreting the study results. Learn more about our Biostatistics and Statistical Programming service.
SWITCH TO OOMNIA NOW.
What our clients say
Read more about the experiences from those who have partnered with us
"All jobs, both large and small, are performed with the same dedication to quality, detail, innovative thinking, and rigor. It is always a pleasure to work with the Wemedoo team."
"Their dedication to, and support of, our projects has resulted in clear quality deliverables. They are always there to answer any questions with expertise in a timely manner. I am looking forward to our next project together."
Frequently asked questions
Get the answers you need about oomnia RTSM
What is oomnia RTSM and how does it improve your clinical trial?
oomnia RTSM (Randomization and Trial Supply Management) is a component of our unified clinical trial software that manages the randomization of participants and the distribution of trial supplies. It improves clinical trials by ensuring unbiased participant allocation, efficient supply management, and adherence to the study protocol, thereby enhancing the integrity and reliability of trial results.
What are the key features of oomnia RTSM?
Key features include automated patient randomization, management of study medication and materials, blinding, data integration and reporting, and compliance and blinding security. These features ensure efficient trial management and optimal resource utilization.
How does oomnia RTSM enhance clinical trial efficiency?
By automating and streamlining randomization and supply management processes, oomnia RTSM reduces manual errors, saves time and ensures that trial sites are adequately stocked with necessary supplies, all of which significantly boost overall trial efficiency.
What benefits does oomnia RTSM offer in terms of Supply Chain Management in clinical trials?
The system offers real-time monitoring of supply levels, automated resupply triggers and efficient management of inventory across trial sites. This ensures that there are no disruptions due to supply shortages, thereby maintaining trial continuity and integrity.
Can oomnia RTSM be unified with other clinical trial management systems?
How can I book a demo and experience oomnia for myself?
In order to book a demo call with an oomnia expert, simply press the button below, select a date in the booking tool and fill in all necessary information. After that, we will send you an invitation.
Explore related tools
Uncover the synergy of solutions tailored to your success